Pigment Color Background
Have you ever wondered how certain compounds get their unique colors? It all has to do with the way that light interacts with those compounds.
When light hits a compound, some of the light is absorbed and converted into heat, while the rest is reflected or transmitted back to our eyes. The colors that we see are actually a result of this absorption and reflection of light. This process is known as subtractive color, and it’s how pigments give rise to color.
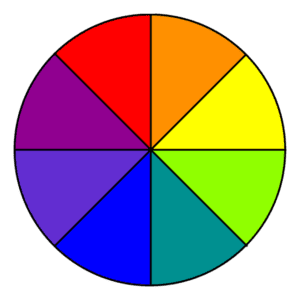
But how do we know which colors a particular compound will absorb or reflect? One helpful tool is the color wheel. If we look at a color wheel, we can see that the colors opposite each other are complementary. This means that if a compound absorbs a certain color of light, it will appear as the complementary color on the color wheel. For example, if a compound absorbs orange light, it will appear blue to our eyes.
Let’s explore a few types of red pigments that have been used throughout history.
Red Ochre – Hematite
Are you ready to discover the world’s oldest pigment? Ochre has been used by humans for over 300,000 years and it’s still widely used today. But what makes this pigment so special?
Ochre is made up of iron-hydroxide or oxide compounds, with the oxidation state of iron always being +3. The variations in color are due to the amount of hydroxide or oxide present, the presence of water, and the size of the crystal particles. Red ochre, in particular, is made up of the chemical formula Fe2O3 and is known as the mineral hematite. This stunning red pigment can be found in rocks and soils all over the globe and gets its vibrant color from a charge transfer electronic transition between oxygen and iron.
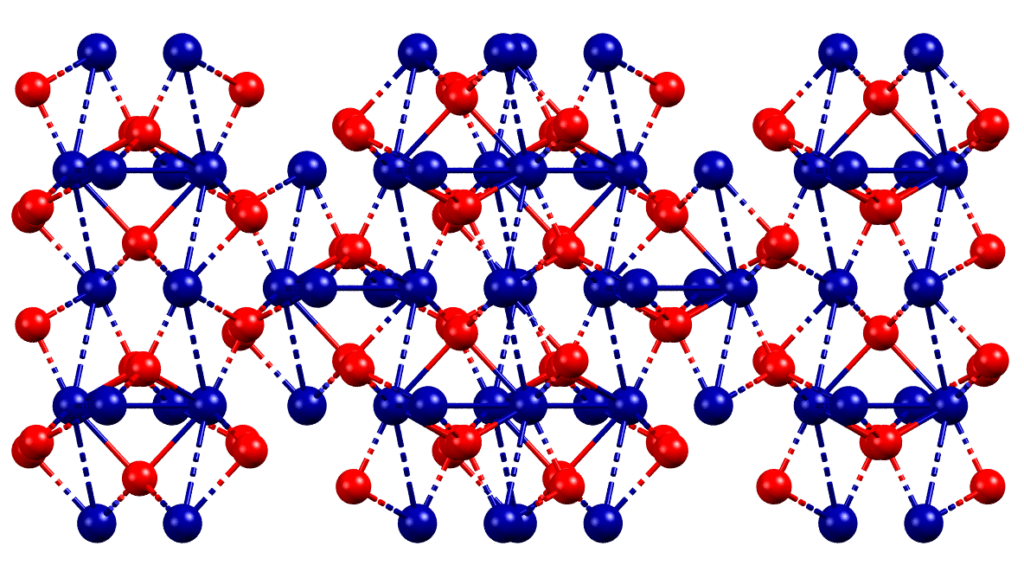
The industrial process for producing red ochre was first developed by the French scientist Jean-Étienne Astier in the 1780s.
Astier’s process involved extracting clay from mines and washing it to separate the sand from the ochre. The ochre was then dried and crushed, and classified by its color and quality. This process allowed for the production of large quantities of red ochre for use in a variety of applications.

Today, modern ochre is often made using more advanced processing, which allows for the production of a product of higher quality and consistency than material that is mined. This type of iron oxide can be more precisely controlled in terms of its color, particle size, and purity, resulting in a more consistent and reliable product.
Despite the advancements in the production of new types of red pigments, red ochre is still highly valued for its natural beauty and unique properties. It is widely used in a variety of applications, including paints, ceramics, and textiles.
Vermilion
Vermilion has been used for centuries as a pigment for painting, dyeing cloth, and adorning objects. The earliest recorded use of vermilion dates back to 8000 BC, when Neolithic peoples in modern-day Turkey used it to adorn their bodies and objects of cultural significance. These early users extracted the pigment from ground cinnabar, a bright red material composed of mercury sulfide that is found in areas with recent volcanic activity or alkaline hot springs.

Vermilion can have several brilliant, opaque colors depending on the way the pigment is processed. Colors can range from bright red to a reddish-purple. The particle size of the pigment determines its color. While vermilion has long been prized for its vibrant color, it is also a highly toxic pigment. It is derived from cinnabar, which contains mercury, a highly poisonous heavy metal. Inhaling mercury vapor or coming into contact with mercury through the skin can cause serious health problems, including tremors, memory loss, and kidney damage. This has made mining cinnabar and producing vermilion a dangerous and highly regulated process.
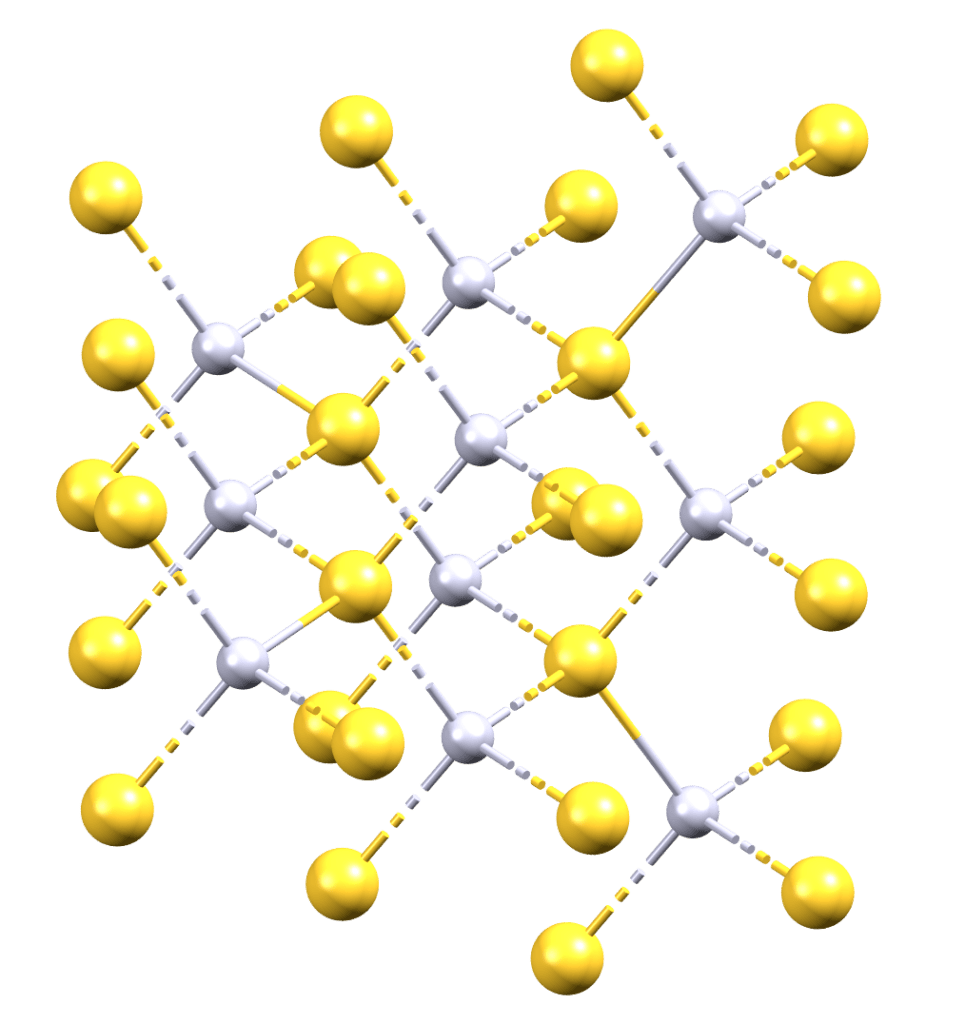
Several cultures developed methods to produce a synthetic version of vermilion, which involved taking mercury and mixing it with sulfur. A black mercury sulfide compound will form and then this is to be heated and vaporized. Upon condensation, the material will crystallize as a black mercury sulfide compound. This can be collected and ground to a fine powder to give the vibrant red color that is desired.
Red Lead
Red lead, also known as minium or triplumbic tetroxide, is a vibrant red pigment that has been used for centuries to add color to paints, inks, and other materials. The history of red lead dates back to ancient times, when it was first discovered by ancient civilizations in Europe and Asia.

It is not clear exactly when red lead was first discovered, as the precise history of this pigment has been lost to time. However, the use of lead compounds as pigments dates back at least to the ancient Romans, who used lead-based pigments to color their clothing, jewelry, and other decorative items.
The Roman author Pliny the Elder referred to red lead as flammeus and described the process of making it in his Natural History, which was written in the 1st century AD. According to Pliny, red lead was made by heating lead oxide (also known as litharge) to high temperatures, which caused it to decompose and form a bright red compound called minium. This process has remained largely unchanged over the centuries.

In addition to its use as a pigment, red lead has also been used for a variety of other purposes throughout history. The pigment was mixed into linseed oil to produce a durable paint to protect exterior finishes.
Inhaling red lead can lead to respiratory problems, such as coughing and difficulty breathing. Ingesting red lead can cause digestive problems, such as nausea, vomiting, and diarrhea. Skin contact with red lead can cause irritation and redness. These symptoms were so common for painters that they were referred to as painters colic and plumbism.
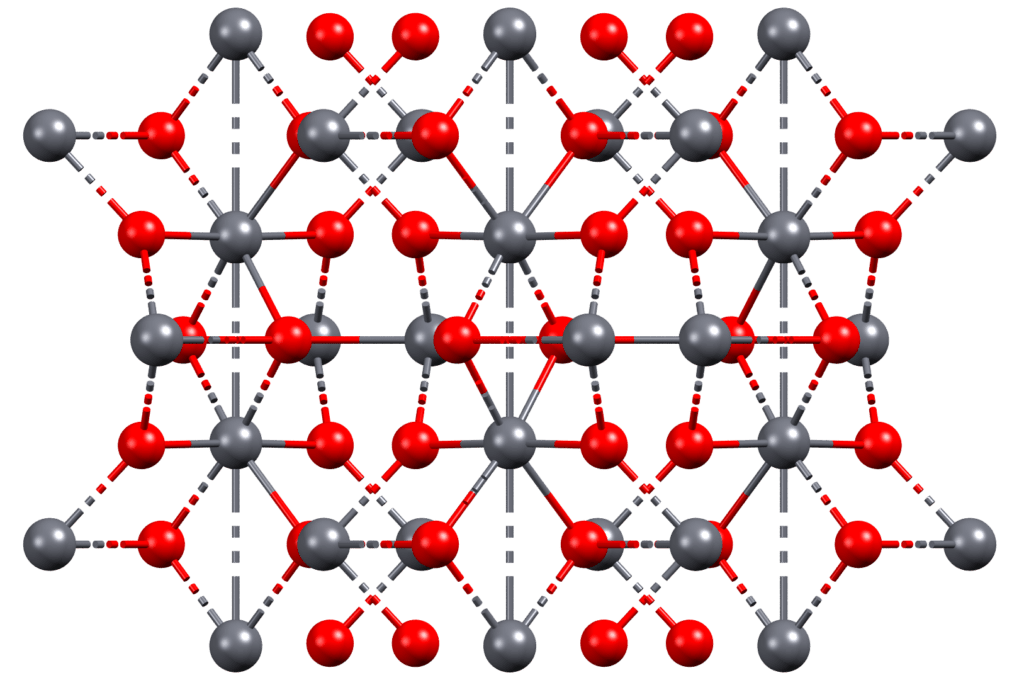
Despite its potential dangers, red lead remains an important pigment in the world of art and design, and it continues to be be used today.
Cadmium Red
While vermilion is a traditional pigment that has been used for centuries, cadmium red is a recent development in the world of red pigments. First discovered in the 19th century, cadmium red quickly became popular due to its bright, vibrant color and excellent resistance to fading.
Cadmium metal was discovered in 1817 by the German chemist Friedrich Stromeyer, who was studying the mineral calamine (zinc carbonate). Stromeyer discovered that calamine contained a small amount of a previously unknown metal, which he named “cadmium” after the Latin word “cadmia,” meaning “calamine.”
The discovery of cadmium metal led to the development of several new pigments, including cadmium yellow and cadmium red. These pigments were highly prized for their bright, vibrant colors and excellent lightfastness, and they quickly became popular among artists and designers.
In addition to cadmium yellow and red, the discovery of cadmium metal also led to the development of other pigments, such as cadmium orange, cadmium green, and cadmium purple. These pigments are widely used in a variety of applications, including paints, inks, and plastics.
The key to Cadmium red is the mixture of Cadmium Sulfide and Selenides which were commercialized in 1910. This pigment is made by heating cadmium sulfide, sulfur, and selenium to 600 degrees Celsius.

In the early 20th century, cadmium red was embraced by artists such as Henri Matisse, who was known for his love of bright, vibrant colors. Matisse used cadmium red extensively in his paintings, and it is likely that his work helped to further popularize the pigment among other artists.

Alizarin
Now that we’ve explored the vibrant world of red lead, let’s turn our attention to another classic red pigment: alizarin. Alizarin is a bright red pigment that is widely used in paints, inks, and other materials, and it has a long and fascinating history.
Madder is the plant from which alizarin, a bright red pigment, is extracted.
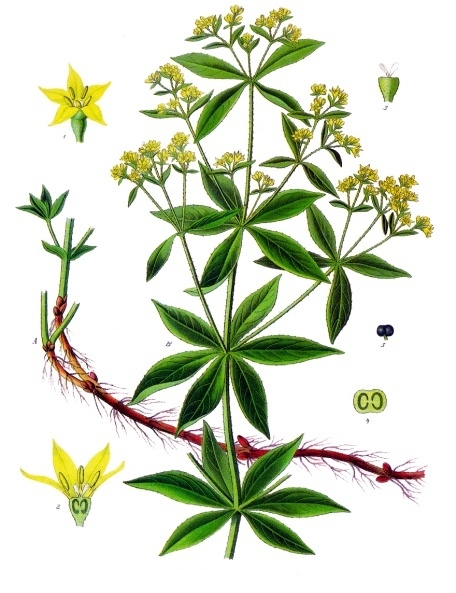
The ancient history of madder, dates back at least to ancient Egypt, where it was used to produce a red dye for textiles and other materials. Madder is native to the Mediterranean region and Asia, and it has been cultivated for centuries for its ability to produce a bright, red dye.
In ancient times, madder dye was produced by extracting the pigment from the roots of the madder plant. The roots were dried and ground into a powder, which was then mixed with water to form a dye bath. Fabric or other materials were soaked in the dye bath to absorb the pigment, which gave them a bright, red color.
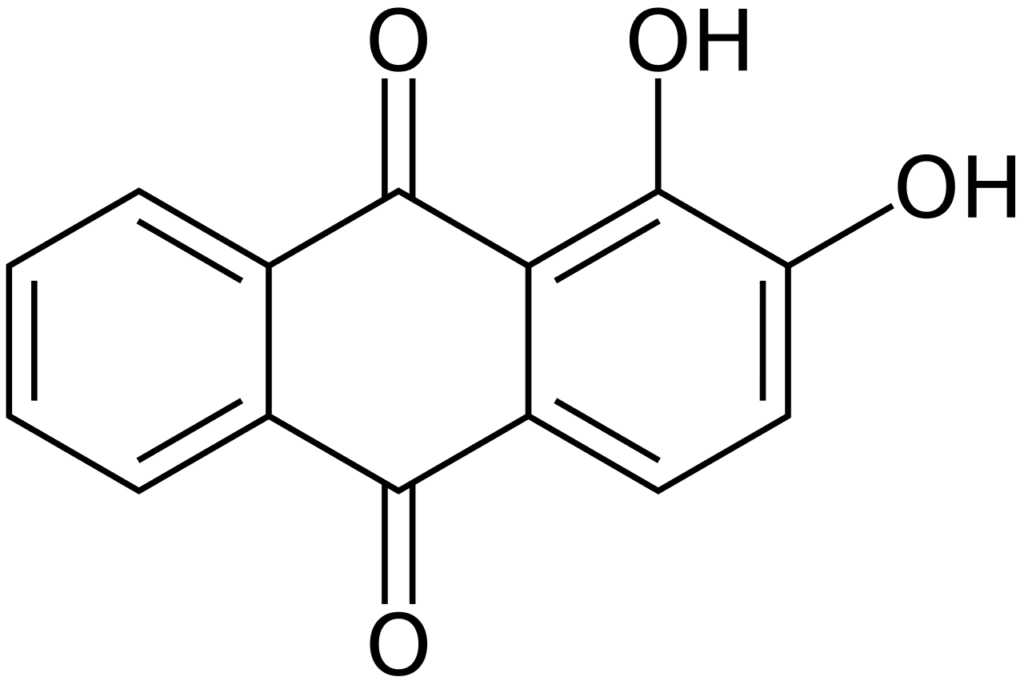
The bright red color of madder dye is due to the presence of alizarin, a pigment that is found in the roots of the madder plant. Alizarin is a member of a group of compounds known as anthraquinones, which are responsible for the color of many natural dyes.
Madder dye was highly prized in ancient times due to its brightness and durability. It was used to color clothing, tapestries, and other decorative items, and it remained popular for centuries.
The bright red color of alizarin is due to the presence of several chemical groups within its molecular structure, including an aromatic ring and a quinone group. These groups absorb light in the blue and green wavelengths, which causes the pigment to appear red to the human eye.

Today, madder is still used to produce a red dye, although it has largely been replaced by synthetic alternatives. Despite this, madder remains an important plant in the history of dye production, and it continues to be used in some traditional dyeing techniques.
Synthetic Alizarin was first discovered in 1868 by the German chemists Carl Gräbe and Carl Liebermann, who isolated the pigment from the roots of the madder plant. They named the pigment alizarin, after the Arabic word for madder, “al-lisari.”
Alizarin quickly became popular as a pigment due to its bright, vibrant color and good durability for the time period. It was widely used by artists and designers to add color to their creations, and it played a key role in the development of the Impressionist movement.
Today, alizarin is still widely used as a pigment, although it has largely been replaced by synthetic alternatives, which are easier to produce and more consistent in color. Despite this, alizarin remains an important pigment in the world of art and design, and it continues to be widely used today.
Cochineal
After exploring the vibrant world of alizarin, let’s now turn our attention to another classic red pigment: cochineal. Cochineal is a bright red pigment that is produced from the dried bodies of the cochineal insect, which is native to Central and South America.
Cochineal has a long and fascinating history, and it has been used as a dye and pigment for centuries. The ancient Maya and Aztec civilizations were some of the first to use cochineal, and they valued it highly for its bright, vibrant color.

Cochineal is produced by grinding the dried bodies of the cochineal insect into a fine powder, which is then mixed with a binder to form a pigment.
The bright red color of cochineal is due to the presence of carmine which is an aluminum salt of carminic acid. This compound is responsible for the red color in the pigment cochineal, and they are present in the dried bodies of the cochineal insect in high concentrations.
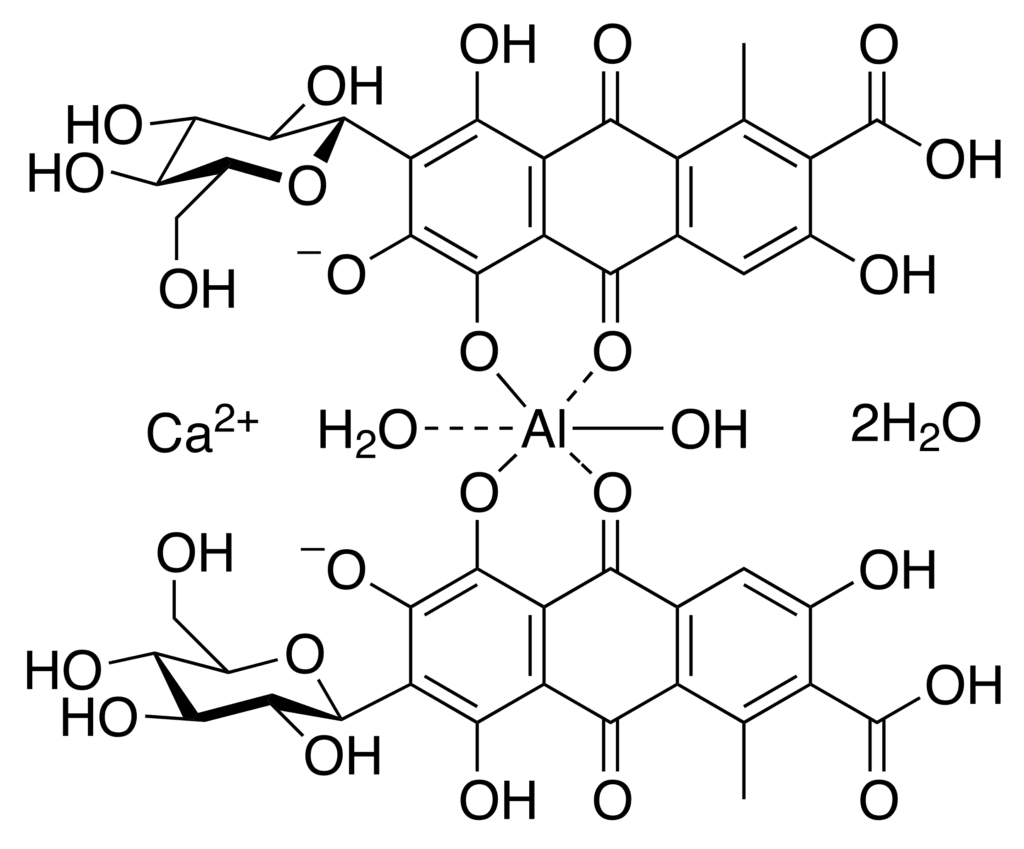
One of the earliest known uses of carmine was as a dye for textiles. The ancient Maya and Aztec civilizations were some of the first to use carmine as a dye, and they valued it highly for its bright, vibrant color. Carmine was used to dye clothing, tapestries, and other decorative items, and it remained popular for centuries.
Carmine has also been used as a pigment in paints, inks, and other materials. It is known for its bright, vibrant color and excellent lightfastness, and it has been used to color a wide variety of materials, including fabrics, paints, and cosmetics.
Carmine has a number of other historical uses as well. It has been used as a food coloring, and it is still used today to color a variety of products, including ice cream, candy, and other sweets. It has also been used as a biological stain, and it is still used today to highlight certain structures in microscopy.
However, being that it is an organic pigment, it is not as durable to light as some inorganic pigments. These carbon based compounds are susceptible to degradation when exposed to light, heat or other environmental factors.
Closing
From the ancient roots of red ochre, vermilion, and madder to the modern-day discovery of cadmium red, red pigments have played a vital role in the history of art and design.
Each red pigment has its own unique properties and characteristics, and each one has contributed to the rich tapestry of color that is available to artists and designers today. Whether used to create bold, vibrant works of art or to add a splash of color to everyday products, red pigments are an essential part of our visual world.